TAAB'S Research Wing
A case study on efficacy and safety of 7% benzocaine wipes in premature ejaculation
The clinical study conducted by Mor Medics Science & Technologies evaluated the efficacy and safety of 7% Benzocaine wipes on 12 male subjects with a history of premature ejaculation (PE).
The primary objective was to assess the efficacy of the product by measuring the intravaginal ejaculatory latency time (IELT) using the stopwatch method.
Secondary objectives included excipient stability, toxicity, sexual function, safety, and consumer acceptance.
The findings of this study suggest that 7% Benzocaine wipes are effective in significantly prolonging IELT and addressing premature ejaculation.
The rigorous study design, including randomization and blinding, enhances the reliability of the results. Furthermore, the use of validated outcome measures and bioanalytical methods strengthens the scientific validity of the study.
The observed increase in IELT and responder analysis results underscore the clinical significance of Benzocaine wipes in improving sexual function and satisfaction among individuals with premature ejaculation.
Colorimetric Assay for Determination of Glutathione Concentration in Cells—a comparative study analyzing the cell permeability of Liposomal Glutathione (L-GSH) vs. Reduced Glutathione (GSH)
Cell permeability of Liposomal Glutathione (L-GSH) vs. Reduced Glutathione (GSH)
A case study investigating the effects of synthetic and natural ascorbic acid on a plasma cell line
The research aims to evaluate how different forms and concentrations of ascorbic acid affect biological processes in plasma cells over specified time periods and pH environments. By exploring these factors, the study seeks to elucidate potential implications for cellular function, including aspects related to collagen synthesis, antioxidant activity, and immune response modulation.
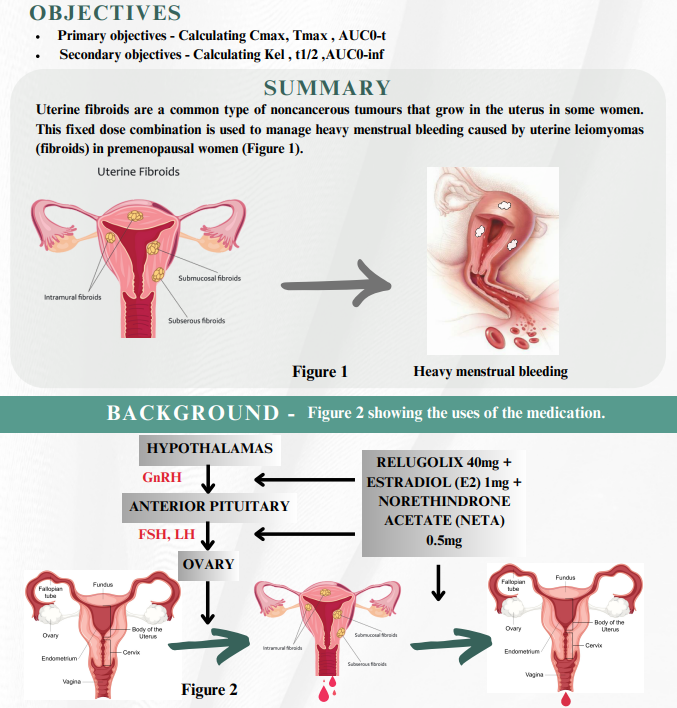
A case study associated with heavy menstrual bleeding
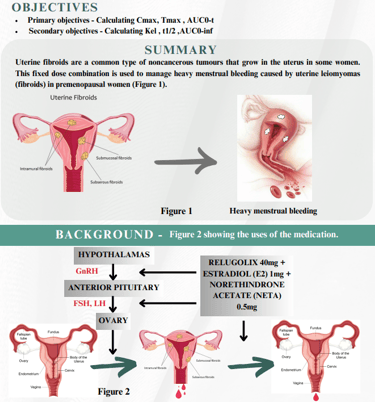
A randomized, open label, two treatment, two period, two sequence, single dose, crossover, comparative, bioequivalence study of Relugolix 40mg + Estradiol (E2) 1mg + Norethindrone Acetate (NETA) 0.5mg with marketed sample of a tablet containing Relugolix 40 mg + Estradiol 1 mg + Norethindrone acetate 0.5 mg of healthy Postmenopausal female, adult, Indian human subjects under Fasting condition.
This combination works by suppressing ovarian hormone levels. In clinical trial Relugolix, Estradiol & Norethindrone substantially reduced menstrual bleeding and improved several others symptoms in woman with uterine fibroids - associated with heavy menstrual bleeding. On the basis of the pharmacokinetic parameters studied, hence TAAB concluded that the Test preparation is bioequivalent with the reference preparation.
Unlocking the Bioavailability Puzzle of Curcumin at the TAAB labs
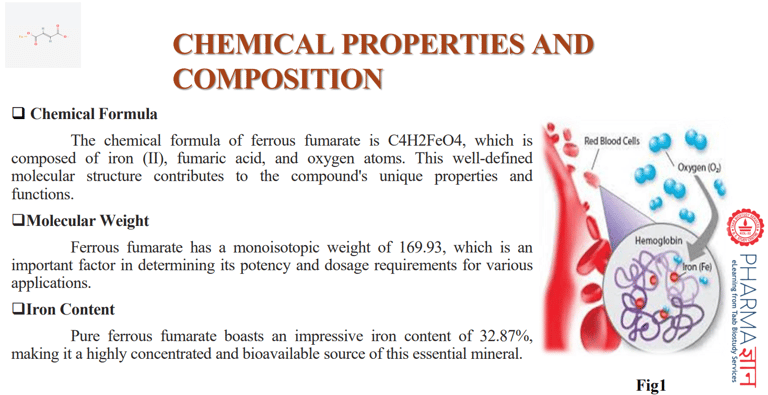
A case study comparing the percentage of ferrous fumarate permeable through cell membranes in test and reference samples
The study demonstrated that ferrous fumarate can effectively promote cellular iron uptake and utilization, suggesting its potential as a valuable iron supplement. The successful outcomes of the plasma cell line study pave the way for further investigations into the broader applications of ferrous fumarate in various health and medical contexts.
TAAB Biostudy Services has been recognized as a Star at the ME HR & Learning Awards 2024 under the prestigious category of "Best Provider for Technical Bio Studies Training"!